Orthopedic Medical Solutions
From Concept to Sales & Everything In-Between
Contract Manufacturing
Our first contracted manufacturing product is a marrow stimulation system called SmartShot which is a device used during orthopedic procedures.
Fulfillment
We do distribution and fulfillment of the Mahe Medical product lines that include things like distal place, spine screws, screw removal kits, frag (fracture kits), etc – all in the orthopedic fields.
Tray Reprocessing
DMTI can manage your surgical trays keeping them traced, stocked, and available when needed. Contact us for more information.
Vertical Integration
Support from OEM manufacturing to testing, all the way to sales
Quality Focus
Mitigating your risk by assuring our quality system is the best-in-market
Customer Service
The ability to speak to a real, live human when you need to, our team is your team

Location & Facility
Located in a university community with access to highways, rail and air service
A Comprehensive Portfolio of Success
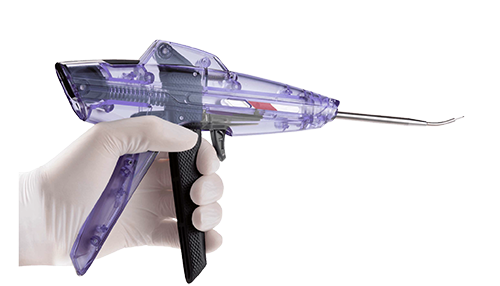
SmartShot®
DMTI has been providing Contract Manufacturing for Marrow Access Technologies since 2019. Their cutting-edge device is the latest first-line treatment for getting patients with cartilage defects back in action.

Xtracta-Screw
DMTI has been providing Contract Manufacturing for Mahe Medical USA since 2020. Their newest device, the Xtracta-Screw, is the latest first-line screw extraction device that alleviates the need for further sterilization after use of the single product.

Welcome to
Disruptive MedTech
Manufacturing Solutions
The products are designed and developed by our customers. DMTI focuses on design improvements around cost, quality, manufacturability and testability (and regulatory compliance, of course).

How We Serve
Our customer’s customer are all the major medical hospitals, ASCs, private practice clinics, the military, the veteran’s administration, etc.
Current Work
DMTI started initial manufacturing with the support of the Wyoming Technology Business Center in 2019. Our team continued to build on the current medical device manufacturing with the addition of distribution/fulfillment of Mahe products (over 1 Ok are FDA approved already) in April of 2020.


Tested & Proven
Medical Manufacturing Excellence
As we realized the desire of hospitals and ambulatory surgical centers (ASC) to work directly with the manufacturers of their implantable devices, it was clear there was a massive gap in the market, that, with the right infrastructure and expertise, DMTI could fill. Additionally, we are continuing our sales partnership with Mahe Medical. We can help clients develop, manufacturer, test, and sterilize; with a proven sales outlet already in place. It’s a great win-win-win scenario. To do that we are focusing the company in the Orthopedic surgical world. The focus is on trauma and then will move into nails and joints (hips, knee, and spine) as we grow. We have visions for a full-service sterilization and medical training operations that will round out our new Center for Medical Manufacturing Excellence.
DISRUPTIVE MEDTECH RECEIVES $50K WBC GRANT
© John Burke via Flikr [Laramie, Wyoming – Disruptive MedTech (DMTI) was recently awarded a $50,000 grant from the Wyoming Business Council. The grant is part of the WBC Coronavirus Business Interruption Stipend offered to assist small businesses during the economic...
DISRUPTIVE MEDTECH AWARDED ISO 13485 CERTIFICATION
[Laramie, Wyoming — Disruptive MedTech (DMTI), a provider of custom, innovative, private branded medical devices, and medical device manufacturing, today announced that the company has received ISO 13485:2016 certification. This certification indicates the company’s...